How Is the Reaction 2h2 O2 2h2o Described in Words
One molecule of hydrogen peroxide is oxidized to oxygen and the second molecule of hydrogen peroxide is reduced to water. The reaction 2 H 2 O 2 2 H 2 O O 2 is a disproportionation reaction.

Chemical Reactions Ppt Download
Click card to see definition.

. Make sure that this guarantee is totally transparent. 2H2 O2 2H2O What does the word product mean in a chemical reation. How is a reaction described when a chemical reaction uses up energy.
Two molecules of hydrogen react with one molecule of oxygen to form two molecules of water. You know that you start with 100 g of hydrogen gas nad 150 g of oxygen. Sodium water -- sodium hydroxide hydrogen gas.
Tap card to see definition. 2H2O22H2O H-484KJ Which answer best describes the transfer of heat that occurs when 141 mol of H2 reacts with 0671 mol of O2. Click again to see term.
Calculate ΔU using ΔU ΔH - PΔV. Methanol CH3OH which can be used as a fuel can be formed by the reaction between carbon monoxide and hydrogen as depicted in the following balanced equation. 2H2 g O2 g 2H2Ol Notice that you have a 21 mole ratio between hydrogen gas and oxygen gas.
This means that regardless of how many moles of oxygen gas you have the reaction needs twice as many moles of hydrogen gas in order to proceed. The chemical reaction 2 H 2 g O 2 g 2 H 2 O g would be expressed as. 2H2 O2 ---.
Hydrogen reacts with the oxygen in the air. Chemical name is hydrogen peroxide. Each paper is composed from scratch according to your instructions.
Hydrogen react with oxygen to form water as per the equation below. Hydrogen plus oxygen produces water. 2H2Ol 2H2g O2g.
The following reaction occurs 1000 atmosphere 2980 K. Consider the following reaction. 2H2 O2 2H2O.
In a chemical reaction a product is the compound or elements that are produced by the reaction. Which substance is completely consumed in a chemical reaction. 2C2H23O24CO2H2OHeat rasaynik abhikriya Kay aahe.
2HgO-- 2Hg O2 Describe in words the chemical composition of the molecules involved and the reaction represented by the equation. Hydrogen gas oxygen gas steam. Word Equation Examples.
It is then checked by our plagiarism-detection software. What is the universal solvent because it can dissolve more things than any other substance. This describes us perfectly.
In the reaction described by the word equation. In the electrolysis of water how many grams of oxygen gas will be produced for every gram of hydrogen gas formed. Describe in words the chemical equation below 2H2 O2 -- 2H2O Two molecules of hydrogen gas two hydrogens bonded together are combined with one molecule of oxygen gas two oxygen atoms bonded together in order to form two molecules of water two hydrogen atoms bonded to one oxygen atom.
2H2 CO CH3OH Suppose 356 g of CO and 65 g of H2 are mixed and allowed. Use oxidation states to identify the element that is being oxidized and the element that is being reduced in the redox reaction Sn 4HNO3 --- SnO2 4NO2 2H2O I see that SN is oxidized 0 -- 4 and book says. This is a combination reaction.
Tap again to see term. 2H202-- 2H2O 4 molecule Hydrogens reacts to 2 molecule Oxygen to yield 2 molecules of water. Here is the equation.
This reaction can be described in words as follows. Could someone please help me start this problem. As a word equation or as Hydrogen and oxygen react to form water or Water is made by reacting hydrogen and oxygen.
2 water molecule 1 oxygen atom 2 hydrogen molecule. Both a- sodium hydroxide and C- water is a reactant. CH3OH l 32 O2 g CO2 g 2H2O l 5000 g of CH3OH and excess oxygen.
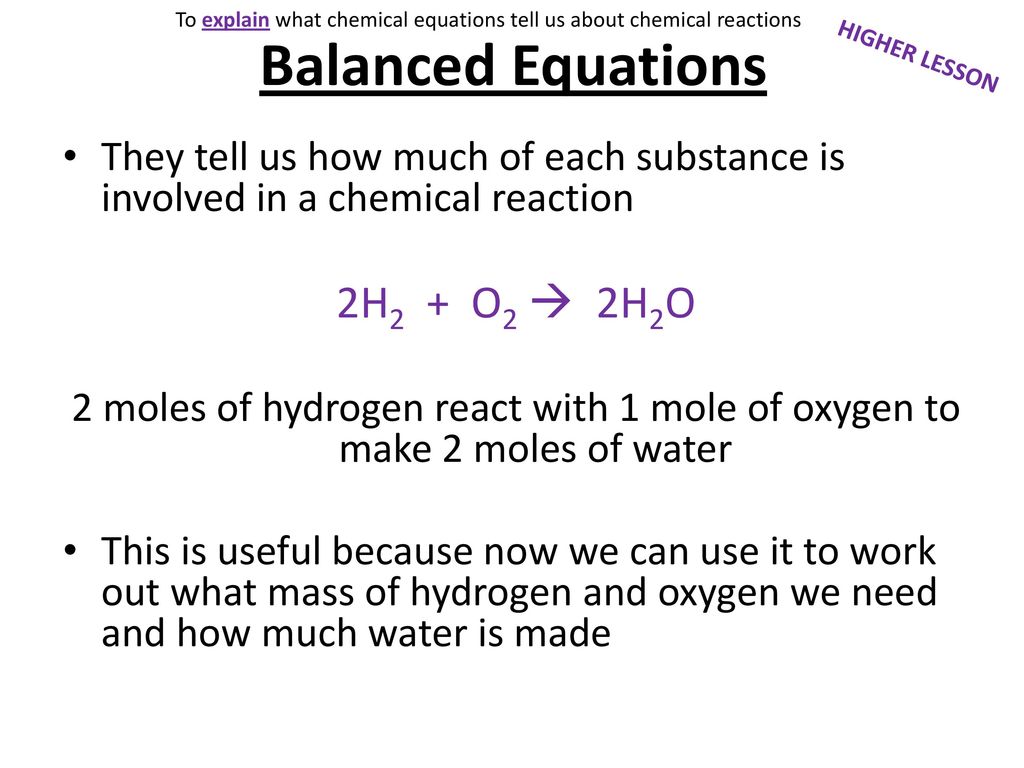
Balanced Equations 2h2 O2 2h2o Ppt Download

Balanced Equations 2h2 O2 2h2o Ppt Download

The Following Is A Partial Chemical Equation 2h2 O2 What Can You Infer About The Products Of Brainly In
No comments for "How Is the Reaction 2h2 O2 2h2o Described in Words"
Post a Comment